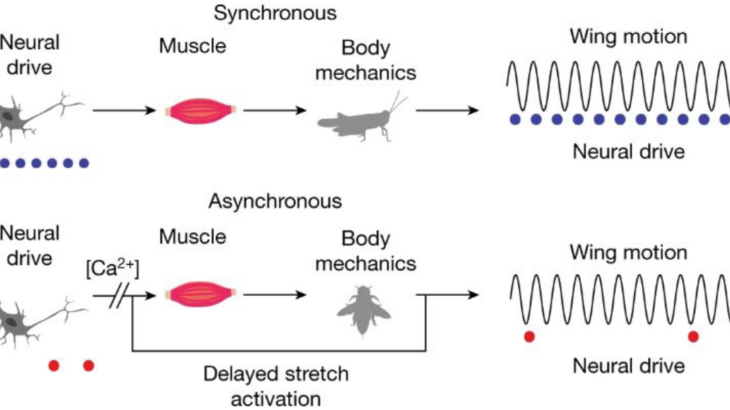
Slow-flapping (also called synchronous) insects use their nervous system to tell the wings how fast to flap. This is how your muscles work as well – muscle contraction is synchronous with a neural signal to the desired muscle. Unfortunately, this mode of actuation does not scale past 100 contractions per second, due to fundamental tradeoffs between force and velocity in muscle. But many insects including flies, bees, beetles, and mosquitos flap their wings faster than 100 Hz. How do these insects break through this neuromuscular speed limit?
It turns out that they have evolved specialized flight muscle that produces force in response to stretch, enabling contraction to be decoupled from the nervous system. These fast-flying insects are called asynchronous fliers, since one signal from the nervous system to the flight muscles may be accompanied by 10 or 20 wingbeats! Therefore, bumblebee wingbeat frequencies are totally emergent. That is, they are not prescribed by the nervous system and emerge from interactions between the muscle, exoskeleton, wing, and fluid environment.
In this project, we are interested in how asynchronous flight has evolved, and whether transitions between synchronous and asynchronous flight have occurred over evolutionary time. We do this using a combination of phylogenetic methods, isolated muscle physiology, and collaborations with robophysicists and roboticists. Using dynamically-scaled robophysical spring-wing systems, we can test how different actuation and mechanical properties
affect wingbeat dynamics in ways that are not possible in real insects.