Wingbeat frequency in flying insects spans several orders of magnitude; Even just in bombycoid moths, wingbeat frequency can vary from 5 Hz all the way up to 80 Hz or even more. All of these moth species have to accomplish these very different frequencies of locomotion using highly conserved flight musculature and neural constraints, so we wanted to determine how the nervous system generates motor programs which accommodate such drastically different frequencies and behaviors. Specifically, as a species flaps faster can the nervous system get more temporally precise to accommodate the higher frequency, or do different frequencies of locomotion require totally different mechanics and strategies? We’ve been recording the comprehensive, spike-resolution motor program for flight in tethered individuals from 8+ species, with wingbeat frequencies ranging from 13 to 50 Hz.
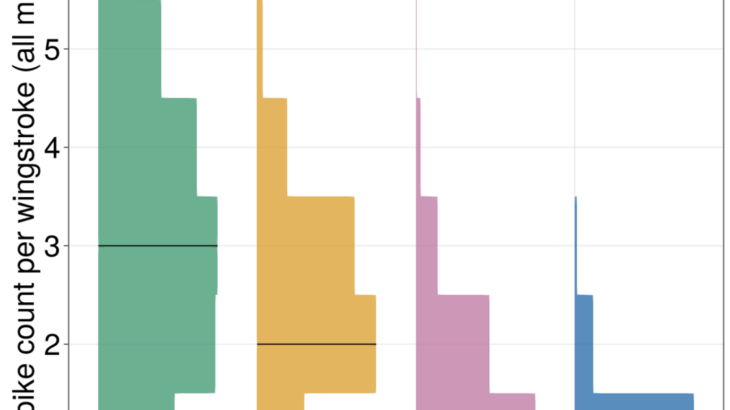
So far we’ve found that within and across species, spike count decreases with increasing wingbeat frequency, and coordination patterns of the motor program and spike timing variance scale differently than naive phase scaling would predict, indicating differing strategies for flight muscle coordination as frequency increases. Using information theoretic techniques, we’ve found that spike timing precision for all muscles in all species is constant at ~1 ms, indicating that faster species do not get commensurately more temporally precise motor programs. This evidence together indicates that absolute timing, more than phase, is preserved as frequency increases. We’ve even been connecting these results with measurements of muscle physiology, showing how the shift to higher frequencies may happen more from changes to muscle properties than from increases in neural precision. As a complete picture, our evidence indicates that to locomote at higher frequencies, animals cannot play the same motor program faster; instead, different frequencies of locomotion require unique neuromechanical strategies.